|
|
PRINT » |
|
|
E-MAIL THIS PAGE » |
|
|
CLOSE THIS WINDOW » |
Key Points of the Bottling Process
Study at 17 wineries identifies impacts on total package oxygen
Bottling is a complex and delicate step of the winemaking process that requires a lot of professional expertise. Maintaining wine quality during bottling, storage and shipping is one of the highest priorities, and the objective is clear: protect the wine from oxidation as long as possible.
Implementation of good bottling practices begins with preparation adapted to the type of wine and the marketing channel. It continues with careful monitoring of the bottling line and, most important, the oxygen intake (pickup).
To obtain a full assessment of oxygen pickup, instrument manufacturers and wine experts have undertaken new efforts to measure total package oxygen (TPO),1 which is the sum of dissolved oxygen (DO) and headspace oxygen (HSO) in the bottle.
Measuring HSO can be achieved using a needle that goes through the closure and is directly connected to an analyzer.2 An alternative method consists of estimating TPO by measuring DO only after shaking the bottle to obtain equilibrium of oxygen pressure between the headspace and wine.3
With the development of non-destructive analytical instruments (PreSens, NomaSense), a complete assessment of the bottling process is now possible. Luminescence-based technologies allow for non-invasive measurement of dissolved oxygen and headspace oxygen through the glass bottle wall.4
Oxygen can dissolve into the wine at every stage of the bottling process, and it can have an effect on wine composition, shelf life and consumer acceptance. During bottling, wine undergoes multiple operations: pumping, filtration, filling and corking or capping. These operations are particularly conducive to the dissolution of oxygen in wines.
At each transfer, and with each treatment, oxygen may penetrate and dissolve in the wine, with an average pickup (introduction) of 1.6 mg/L.5 As wine progresses through bottling, a so-called “U-curve” of dissolved oxygen occurs. High amounts of oxygen are introduced at the beginning and end of the bottling process, and lower and steady levels are common in the middle of a bottling lot.6 According to Zoran Ljepovi?, Constellation Brands quality assurance director, the bottling process should start with a DO of less than 1 mg/L, and if any increase occurs throughout the run, it should be maintained below 0.3 mg/L.7
At filling, oxygen concentration in bottle headspace can range from 1.5 to 2.5 mg/L.1 The oxygen concentration depends on three factors: wine volume, headspace volume and oxygen concentration in the headspace. Thus, at the end of the packaging process, it is possible to find wines with dissolved oxygen levels ranging from 2 to 4 mg/L and a final oxygen content approaching 8 mg/L.8
Measurement of total package oxygen
Dissolved in excessive amounts, oxygen can cause irreversible changes in the color of the wine and its flavor profile after packaging. The influence of oxygen on red wine aroma compounds and sensory properties has been more difficult to confirm compared to effects on wine color. In a two-year Cabernet Sauvignon wine closure trial, M.J. Kwiatkowski showed that, with larger headspace, significant losses of SO2 are observed soon after bottling, and wines developed a higher oxidized aroma score.9
White wines contain lower levels of polyphenols than red wines (0.2 to 0.5 g/L), mainly hydroxycinnamic acids, but these remain very important for oxidation and hence contribute to browning and loss of varietal aroma.10
Measurements can be performed in real time using specific equipment for assessing oxygen at critical steps (transfer, filtration, bottling and storage). These measurements can be performed directly on the transfer lines and in the bottles (dissolved oxygen in the wine and gaseous oxygen in the headspace). Ken Fugelsang, professor emeritus of enology at California State University, Fresno, recommended TPO levels below 1.25 mg/L in bottled red wines and below 0.6 mg/L for white and rosé wines at bottling.11
In general, a winemaker should target a maximum of 1.0 mg/L of TPO at bottling, but this can be difficult to achieve. Levels above 2.5 mg/L can be problematic with a considerable effect on wine shelf life and sensory profile. Nomacorc’s research team revealed that the aroma of Chardonnay wines is altered four months after bottling when wines are exposed to more than 2.5 mg/L of TPO.12 The decrease of the fruity aromas in Chardonnay wines was associated with an increase in oxidation, caramel and honey characters.
The ultimate goal of managing TPO during bottling is to reduce bottle-to-bottle variation and provide the same tasting experience for the wine consumer. While most oxygen-management studies have focused on wine aroma and sulfur dioxide consumption, it is obvious that we are lacking information about how the TPO is affected by bottling practices. Several points need to be addressed as to when the most oxygen is dissolved into a wine during bottling as well as how this could be prevented.
To examine the bottling processes that have an impact on TPO, California State University, Fresno, embarked on a collaborative study with Nomacorc.
Bottling audits
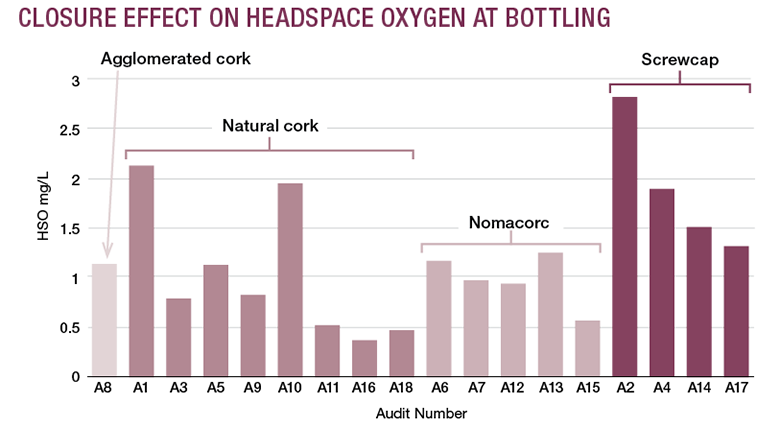
Oxygen has the potential to dissolve into wine at every stage of the bottling process. Different cellar practices can have different impacts on TPO. In order to analyze the possible sources of oxygen intake, bottling audits were conducted at 17 California wineries ranging in production capacity from less than 5,000 gallons per year to approximately 120,000 gallons per year. Eight white wines and nine red wines were analyzed for oxygen uptake during bottling using different closure types: eight lots closed with natural cork, five lots closed with Nomacorc synthetic closures, four lots received screwcaps and one lot received agglomerated cork.
Total package variations were studied based on the following critical cellar practices with impact on oxygen ingress:
• Type of wine
• Type of closure and bottle used
< p>• Bottling tank volume and diameter• Length and diameter of hose between bottling tank and filler bowl
• Number of filling spouts and closure heads
• Bottled volume and line speed
• Technology of filling
• Use of wine to prime the circuit and volume used
• Use of inert gas before the process to maintain inert headspace in the bottling tank, inline, to inert bottles before and after filling, at corking, for purging of caps (yes/no and type), and at the end of a bottling run
• Use of vacuum (and level of vacuum in negative millibar) at corking
The TPO was determined using a NomaSense O2 P300 for dissolved oxygen (DO) and a piercing device for a destructive measurement of headspace oxygen (HSO).
Bottle sampling for DO started from the beginning of the bottling run with samples taken in triplicate at each filling spout. Samples were taken at Bottle 1, Bottle 50, Bottle 150, Bottle 300 and Bottle 500. Samples for HSO measurement were taken in the middle of the run, and DO was measured simultaneously. At the end of the run, samples were taken when the bottling tank was empty, when one-half of the remaining wine reached the membrane, when all remaining wine reached the membrane, when the filler bowl was half empty and from the last three bottles.
TPO variations
Results varied considerably among bottling units, with TPO values ranging between 0.6 and 3.3 mg/L. Among the 18 audits, seven failed to achieve levels below 1.5 mg/L and four had considerable bottle-to-bottle variations, as shown by the error bars in “TPO Variation Mid-Bottling Process.”
With few exceptions where wine DO was high compared to HSO, we observed that TPO at bottling is mostly affected by the gaseous oxygen of the bottle headspace. In some audits, HSO reached levels close to 2.5 mg/L, which agrees with previous observations.8
As the headspace is more saturated with oxygen than the wine, Henry’s law of equilibrium13 will favor the dissolution of gaseous oxygen, leading to an increase of TPO followed by an increase of DO during storage.11 With a 30 mm fill height for screwcap closures, the headspace can supply more than 2 mg/L of oxygen to the wine at a bottling temperature of 68° F.
This may not be a problem if the sulfur dioxide level in the wine is adequate. The optimum level of sulfur dioxide for a wine is a complex function of several variables (pH, DO, oxidants and other wine elements such as carbonyls and polyphenols). As a general rule, 4 mg of free sulfur dioxide is required to neutralize 1 mg of dissolved oxygen. Thus, additional sulfur dioxide would need to be available to combine with the products of oxidation that would generate from the dissolution of the gaseous oxygen during bottle storage.
Survey results indicated several key areas of concern for excess oxygen pickup into wine during the bottling process. Contact with air at the time of bottling can be variable, depending on the care taken and equipment used. The most and least efficient bottling runs are reported in the table “Cellar Practices and TPO." Among the 17 bottling audits, the lowest TPO level was recorded in Audit 11 and the highest TPO level in Audit 2. The two audits differ in both DO and HSO levels and management procedures.
Generally, the type of wine and the hose length did not have a significant impact on oxygen pickup. An inverse relation between speed and oxygen dissolution was instead observed in this study. Slower bottling lines generally dissolved more oxygen than the high-speed units. Slower filling and corking or capping operations mean that the wine stays longer in the line, the filler and the bottle prior to filling, resulting in an increased pickup of oxygen. Bottling line rates should be maintained at sufficient levels to minimize this effect.
Wine transfer and impact on DO
As reported in the table “Cellar Practices and TPO,” the potential for a higher DO level during bottling seems to decrease with the use of inert gas in the bottling tank and transfer line. If any headspace is present in the bottling tank, it is important that the surface is constantly treated with inert gas such as CO2, N2 or argon to prevent oxygen from dissolving into the wine. Protecting the wine from oxygen during the transfer from the bottling tank to the filler is essential to minimize oxygen pickup.
As seen in “U-Curve throughout the Bottling Process,” audits 5 and 18 started with DO levels above 1.5 mg/L, and these levels were maintained throughout the bottling run. Wineries 4, 7 and 11 were able to eliminate the U-curve, maintaining a DO level below 0.5 mg/L.
In the bottling tank, hoses and filler bowl are where the wine is most likely to come into contact with oxygen during pumping. Critical times for the dissolution of oxygen are the beginning and end of the transfer, because this is when the wine is most likely to come in contact with oxygen due to higher turbulence.
In fluid mechanics, it has been proved by Osborne Reynolds that turbulence depends on the mean flow velocity, pipe diameter, density and fluid viscosity.14 A turbulent flow greatly increases the surface area of reaction with ambient air, thereby increasing oxygen dissolution. With a higher transfer flow velocity and frequent pumping, dissolution of oxygen is usually more important, especially if a centrifugal pump is used.
Analysis of audits 4, 7 and 11 reveals good practices able to reduce DO during critical moments of one transfer, which reduces the U-curve effect. Whenever a wine is moved from one container to another, it should be protected with inert gas.
The process of sparging with inert gas is based on the application of Henry’s law,13 which states that the solubility of a gas in a liquid is proportional to the partial pressure of that gas in the gaseous atmosphere in contact with the liquid. During sparging, a partial pressure develops between the inert gas (N2) and the dissolved gas (O2). The difference in partial pressure causes O2 to leave the wine.
According to Henry’s law, many factors would affect the efficiency of sparging. These factors include temperature of the wine, inert gas bubble size, gas pressure, contact time between the gas and wine and the flow rate of gas in relation to the flow rate of wine. Small, inert gas bubbles create a greater interface area with the wine, leading to a more efficient stripping of oxygen. The b ubble size is determined by the porosity of the sintered element in the sparging unit. Iowa State University’s extension enologist Murli Dharmadhikari noted that a maximum bubble size of about 0.03 mm diameter is considered acceptable for sparging.15
Dharmadhikari noted that to displace oxygen, the tanks should be purged with 3 to 7 volumes of CO2. He also suggested a temperature of 59° to 68° F, a pressure of 1 to 2 bars and a flow rate of 0.1 to 0.3 liters of inert gas per liter of wine to improve sparging efficiency. And for removing the oxygen already dissolved in the wine, he also recommended sparging the hose with gas and the wine during pumping.15
Best practices for reducing DO and U-curve effect during bottling are summarized in the table “Best Practices for Managing TPO at Bottling.”
Reducing oxygen uptake during and after filling
At the filling step, oxygen dissolving into wine is due to variation in bottle headspace related to improper fill height, wine temperature, solubility of gases, bottle size and shape.
Failing filler spouts drive air into the bottle, resulting in an improper application of inert gas. The key components regarding filling and how it affects oxygen uptake relate primarily to the turbulence of flow from the filling spouts and the use of vacuum or inert gas during filling.
A rapid, non-turbulent flow will decrease the uptake of oxygen and should be the first priority, even if a vacuum is used to evacuate air from the bottles or inert gas is sparged into the bottles. As observed in audits 1 and 10, even with the use of vacuum and inert gas after filling, the final TPO level in both cases was higher than 1.5 mg/L. There are many other factors probably not controlled for.
When flowing from the spout into the bottle, the wine encounters both stainless steel and rubber surfaces. These should be maintained to be as smooth as possible with regular inspection and, when necessary, re-machining of the filling spouts surface to ensure a desirable laminar flow with proper maintenance of the filler spouts.
Use of inert gas
Inert gas is used to evacuate air from the bottle and/or the headspace above the wine. Nitrogen and carbon dioxide are the gases of choice, as argon has a tendency to increase pressure in the bottle when the wine temperature increases. Compared to nitrogen, CO2 has the advantage of being soluble in wine, which minimizes the pressure produced by the reduction of headspace volume until saturation. This does, however, have the disadvantage of increasing the dissolved CO2 content in the wine. While its presence as a “spritz” may be acceptable in some white wine styles, it is less desirable in red wines.
Moreover, CO2 dissolution can reduce potential oxygen levels immediately and can increase the likelihood of the wine becoming reductive. Consequently, it is recommended that winemakers set low CO2 levels, especially for red and full-bodied white wines. The Australian Wine Research Institute suggests 1 to 1.2 mg/L of CO2 under cork and 0.6 to 0.7 mg/L under screwcap.16
An empty bottle contains approximately 225 mg of oxygen, which can increase DO in the wine by 0.3 to 0.7 mg/L, depending on the filling technology adopted (carousel or orbital).
One way to remove oxygen from the bottle is to purge inert gas prior to filling, although in some cases it appears inefficient, as seen in table “Managing Oxygen During and After Filling." Winemakers recommend a volume of gas seven to eight times that of the bottle, depending on the turbulence and rate of purging.
Total removal of air by purging inert gas is often difficult because the incoming gas causes significant turbulence and mixing as it enters the bottle. Alternately, inert gas can be applied after filling to displace air from the bottleneck. The most effective results are usually achieved by combining these two methods.
It is important to optimize bottling conditions for each closure type and adapt the final headspace pressure to the specifications of each closure/bottle combination. In particular, the limits of using inert gas with a screwcap compared to other closure types are highlighted in this study. The effect of screwcapping on oxygen introduced is more important than with other closure types.
The importance of managing headspace level for screwcaps has been emphasized in scientific literature. If screwcaps are not appropriately purged with gaseous N2, they will compress a higher level of O2 under the cap. E. Dimkou et al. reported the impact of managing HSO for screwcaps on the decline of free SO2 and showed that as lower headspace volume is linked to a screwcap, it is generally associated with higher concentrations of hydrogen sulfide.17
When closing bottles with screwcaps, applying a third dose of inert gas immediately prior to capping can be effective. However, some gas interchange can occur between the gas inside the empty cap and that in the headspace during the application. Empty screwcaps can be purged, but headspace purging is not anywhere close to 100% effective.
Other disadvantages of flushing inert gas prior to capping or corking are that this procedure tends to remove some of the desirable volatile aroma compounds important to wine quality. For this reason, “dosing” of liquid nitrogen on top of the wine in the bottle is favored by some winemakers.
Because liquid nitrogen is a cryogenic liquid (-320° F), it is usually delivered to the bottling line through a vacuum-jacketed pipe to minimize premature vaporization of the liquid. When the liquid nitrogen is introduced into the container, the liquid vaporizes due to a temperature change. The liquid nitrogen injection drives out oxygen and covers the contents in the container with nitrogen gas.
Application of a drop of liquid N2 is efficient because the gas is induced by evaporation, which allows the gas to be expelled from the bottleneck without creating turbulence or air mixing. According to liquid nitrogen suppliers, by introducing a very small dose of liquid nitrogen into the bottle after filling, winemakers reduce HSO content by more than 85%. One part of liquid nitrogen warms and turns into 700 parts of gas, displacing oxygen from the headspace.
Improper application of a liquid N2 drop or insufficient amount during screwcapping could be associated with an increase of HSO. Because the vapor requires more volum e than the liquid it replaces, the pressure within the container is increased.
Applying a screwcap while liquid N2 remains in the headspace could pressurize the headspace and compromise the seal. The liquid N2 should be allowed to fully evaporate before the cap is applied. The time between dosing of the liquid N2 and screwcapping needs to be designed to allow the intended liquid N2 to gas off.
Another option would be that the screwcap is applied but not immediately spun onto the bottle. In this case, it is important that the liquid N2 is allowed to fully evaporate before the cap is sealed to avoid pressure buildup in the bottle.
Pressure-influencing factors include the wine level in the bottle, wine temperature and dose-droplet size. Sophisticated liquid nitrogen injectors use electronic controls to adjust injector dosing to account for line-speed variations. Various nozzle sizes are available to control the droplet liquid volume at various dose durations.
CHART Industries’ engineers have designed automated dosing equipment and complete systems that precisely dispense a measured dose of liquid nitrogen into each bottle prior to sealing.
Improper application can also be responsible for damaged screwcaps, which affect the efficiency of the inerting step and increase oxygen ingress during storage. Proper screwcap application needs to be verified using good quality-assurance practices.
Pressure effect
An important consideration is that leakage, oxidation and wine spoilage can result from excessive gas pressure under the closure. As the wine temperature rises, risks of pressure leak problems increase due to wine expansion. A wine volume increase of 0.166 mL per degree Fahrenheit could be registered.18 As the wine volume changes, the pressure rises and the gas in the headspace is compressed.
With an appropriate fill height and adequate vacuum level to compensate for temperature differences, it is possible to maintain internal bottle pressure at levels equivalent or less than 0.1 bar (2 psi) at 68º F.
Cork manufacturers suggest to fill to the level designated by the bottle manufacturer and to adjust the fill level by 0.55 mm to compensate for every degree Fahrenheit above or below 68º F in cork-sealed bottles.
With a cork closure, the air can escape around the closure to decrease pressure inside the bottle. Wine can also escape under pressure by capillary action, and the cork may be expelled out.
The screwcap is less elastic than a natural or synthetic closure and unable to reduce pressure. Depending on the screwcap, liner and application conditions, screwcaps are typically designed to vent in the range of 30 to 50 psi for wine closures.
It is essential that the level of headspace under a screwcap be maintained greater than the standard 10 to 15 mm level of an internal closure. Most winemakers adopt a level close to 20 to 30 mm for screwcaps with the head pressure at bottling ranging between 0.5 bar and 1 bar based on manufacturers recommendations, although some closures can withhold more than 4 bar.
Use of vacuum
Sparging with inert gas or dosing liquid N2 was, in some audits, less effective at controlling HSO than the use of vacuum. When the air is evacuated by vacuum from the empty bottle, the air is moved inside the filler bowl above the wine and mixed with nitrogen. This application eliminates the turbulence created by injecting gas into the bottle and reduces excessive gas use.
An advantage of evacuating the bottle of air after filling is the removal of oxygen from the headspace and preventing the creation of pressure in the bottle due to the “piston” effect by inserting the closure.
After insertion, pressure in the bottle should preferably be in equilibrium with the atmosphere. The vacuum level can be adjusted to compensate for temperature differences and reduce pressure leak risks. The lack of information in this area makes this procedure less reliable than adjusting the fill level for wine temperature compensation. This method seems less reliable than adjusting fill levels because it places much responsibility on the performance of bottling equipment.
Based on the filler manufacturer’s recommendation, the vacuum should be adjusted at levels close to -500 millibar (-6.75 psi) at the filler and -70 millibar (1 psi) at the corker.
Nomacorc suggests monitoring pressure in the ullage (headspace) approximately 10 minutes after bottling to ensure it is between -300 millibar (-4.35 psi) and 300 millibar (4.35 psi). Rather than using the vacuum pump gauges that may not accurately reflect the actual ullage pressure, the company recommends manual measurement through the closure into the headspace with a pressure gauge attached to a thin needle.19
According to veteran bottling consultant George Crochiere, a proper setup supplying a vacuum at corking can help reduce the amount of oxygen absorption into the wine.20 In this study, the lowest HSO levels were registered when -102 to -135 millibar of vacuum were applied at corking. Increasing the vacuum did not necessarily reduce HSO, as observed in audits 1 and 13, where -677 millibar and -169 millibar were applied respectively. The absence of vacuum or a reduced level (-13.5 millibar in audit 10) was instead associated with an increase of HSO.
Summary of best practices for managing TPO at bottling
Regular measurements of both DO and HSO, preventive maintenance and closure inspection are essential components of efficient bottling. Keeping constant low levels of TPO during bottling is challenging and closely related to good cellar practices.
Key points of the bottling process that have impact on TPO are represented in the illustration on page 43 of the print edition. Some of the best practices to manage DO and HSO during bottling are summarized in the table “Best Practices for Managing TPO at Bottling.”
Hend Letaief was assistant professor of wine chemistry at California State University, Fresno. She earned a master’s degree in viticulture and enology from Montpellier SupAgro in France and a Ph.D. in food science and technology from the University of Turin in Italy. She has eight years of diverse and practical international experience in enology and is the author of “Winemaking Process In Valorization of Winemaking By-Products” and Texture Analysis for the Definition of Wine Grape Quality. Letaief has joined Enartis USA as technical direc tor.
This work was funded by Nomacorc and benefited from the support of the California wine industry.
The author is grateful to Bertille Goyard from AgroSup Dijon and Pauline Martinaggi from ESA Angers for conducting the experiments as well as the Wine Science Forum advisory board and Ashley Heisey for supervising the audits and providing technical support to the project.
References
1. Nygaard, M. 2010 “Oxygen management from grape to glass.” Wine Industry J. 5: 24-28.
2. Vidal, J.C., J.C. Boulet, M. Moutounet and C. Toitot. 2004 “Comparison of methods for measuring oxygen in the headspace of a bottle of wine. “ J. Int. Sci. Vigne Vin. 38 (3): 191-200
3. Vilacha C. and K. Uhlig K. 1984 “Die messung niegriger gesamtsauer-stoffgehalte imabbgefüllten bier. Brauwelt, 18, 754-758
4. Dieval, J. B., M. Veyret, J.C. Vidal, O. Aagaard and S. Vidal. 2009 “Validation of non-invasive measurement of Total Package Oxygen”. In Final Papers of the 32nd World Congress of Vine and Wine CD. Le Bulletin de l’OIV.
5. Karbowiak, T., R.D. Gougeon, J.B. Alinc, L. Brachai, F. Debeaufort, A. Voilley and D. Chassagne. 2010 “Wine oxidation and the role of cork.” Food Sci. & Nutrition. 50: 20-52.
6. Moutounet, M. 2013 “Généralités sur l’importance de l’oxygène dans la vie des rosés.” Revue française d’œnologie. 260: 21-25.
7. Ljepovi?, Z. 2016 “Bottling Prep – Best Practices.” Unified Wine and Grape Symposium, Sacramento, Jan 24-26 2016.
8. Vidal, J.C. and M. Moutounet. 2007 “Suivi de l’oxygène au cours du conditionnement : nouveau critère de l’assurance qualité.” Bulletin de l’OIV. 181: 1-6.
9. Kwiatkowski, M.J., G.K. Skouroumounis, K.A. Lattey and E.J. Waters. 2007 “The impact of closures, including screw cap with three different headspace volumes, on the composition, color and sensory properties of a Cabernet Sauvignon wine during two years storage.” Aus. J. of Grape & Wine Research. 13: 81-94.
10. Singleton, V.L. 1987 “Oxygen with phenols and related reactions in musts, wines, and model systems: Observations and practical implications.” Am. J. of Enol. & Vit. 38: 69–77.
11. Fugelsang, K. 2009 Department of Viticulture & Enology, Fresno State. Personal communication.
12. Letaief, H. 2014 “Impact of Bottling on Wine Quality,” Wine Science Forum, Napa, February 4, 2014.
13. Henry, W. 1803 “Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures”. Phil. Trans. R. Soc. Lond. 93: 29–274.
14. Falkovitch, G. 2011 Fluid Mechanics. Cambridge University Press.
15. Dharmadhikari, M. 1992 A little Wine knowledge Goes a long way In: Ohio Grape-Wine Short Course. Proceedings Horticulture Department Series 630. The Ohio State University. 20:31
16. Stelzer T., J. Grosset, M. Brajkovich, J. Forrest and B. Rankine. 2005 taming the SCREW: a manual for winemaking with screw caps. 99.
17. Dimkou, E.M., J.B. Ugliano, S. Diéval, S. Vidal, O. Aagaard, D. Rauhut and R. Jung. 2011 “Impact of Headspace Oxygen and Closure on Sulfur Dioxide, Color and Hydrogen Sulfide Levels in a Riesling Wine.” Am. J. of Enol. & Vit. 62: 261-269.
18. Boulton, R.B., V. Singleton, L.F. Bisson and R. Kunkee. 1996 Principles and practices of winemaking.
19. nomacorc.com/bottling-guidelines.
20. Crochiere, G.K. 2007 “Measuring oxygen ingress during bottling/storage.” Practical Winery Vineyard. (January/February): 74-84.
|
|
PRINT » |
|
|
E-MAIL THIS ARTICLE » |
|
|
CLOSE THIS WINDOW » |